Abstract
[Background] Small (0.003-1.0%) populations of glycosylphosphatidylinositol (GPI)-deficient granulocytes (Grs) (sPNH-GPs) are often detected in patients with acquired aplastic anemia (AA) and low-risk myelodysplastic syndromes, and are thought to represent a marker of immune-mediated bone marrow (BM) failure. On the other hand, our recent studies revealed that miniscule (<0.003%) GPI(-) Gr populations (mPNH-GPs), which are derived from mono- or oligo-clonal PIGA-mutated hematopoietic stem cells (HSCs), are detectable in the peripheral blood (PB) of 80% of healthy individuals (HIs) and some cord blood (CB) samples (ASH 2021, #2181). The high prevalence of mPNH-GPs in HIs and the similarly high (60%) prevalence of PNH-phenotype Grs in AA patients suggest that the proliferation of PIGA-mutated HSCs may inevitably occur in immune-mediated AA. However, our earlier study failed to detect mPNH-GPs in some AA patients in long-term remission who possessed HLA-class I allele-lacking (HLA[-]) Grs after immunosuppressive therapy (IST) (unpublished data). In patients with long-standing AA, the prevalence of mPNH-GPs may be lower than that in HIs due to the replacement of mPNH-GPs by other aberrant Grs.
[Methods] To test our hypothesis, we examined PB samples from 40 patients who developed AA 1-43 (median 11) years previously and were all negative for sPNH-GPs for the presence of mPNH-GPs. At the time of sampling, 28 including 6 patients possessing HLA(-) Grs were in remission after IST (n=8), thrombopoietin-receptor agonists (TPO-RAs, n=14), anabolic steroids (AS) (n=3) after failing IST, or spontaneous resolution (n=3). Eight patients were refractory to all therapies, 3 of which were transfusion dependent. The remaining 4 with non-severe AA had not been treated. Four patients with pure red cell aplasia (PRCA) who had a 12-32 year history of anemia responding to cyclosporine (CsA) were included in this analysis. PB samples from 93 HIs and 22 CB served as controls, and samples from a patient with chronic myeloid leukemia (CML) were used as a positive control for clonal hematopoiesis. mPNH-GPs were deemed positive when 4 or more CD11bhigh dots that formed tight clusters were present in the FLAER- fraction of Grs that were enriched using microbeads coated with PE-labeled anti-CD55 and anti-CD59 antibodies.
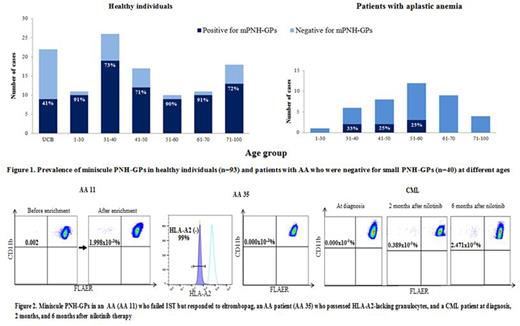
[Results] We first determined the prevalence of mPNH-GPs in HIs (n=93) at different ages. The prevalence was approximately 80% in all different age groups with the exception of the CB, which showed a prevalence of 40.9% (Figure 1). In contrast, mPNH-GPs (0.101-1.998x10-3%) were detected only in 7 (17.5%) of the 40 patients with a long history of AA (Figure 1). These 7 patients were refractory to IST but were in remission at the time of sampling after responding to TPO-RAs (n=3, Figure 2) and AS (n=1); while 3 experienced spontaneous resolution of AA after cessation of treatment. Of the 33 AA patients who were negative for mPNH-GPs, only 8 were in remission, and 5 of the 8 possessed HLA(-) Grs that accounted for 75%-99% (median 96%) of the total Grs (Figure 2). All 4 patients with PRCA who were negative for sPNH-GPs possessed mPNH-GPs, despite the fact that they responded to CsA. The lack of mPNH-GPs in AA patients with complete clonal hematopoiesis with HLA(-) Grs prompted us to examine a CML patient whose Grs were 100% BCR-ABL-positive. mPNH-GPs were undetectable at the diagnosis, but became detectable 2 weeks after the initiation of nilotinib therapy, and increased after 6 months when the patient achieved cytogenetic remission (Figure 2).
[Discussion] The persistence of mPNH-GPs in AA patients who failed to respond to IST suggests that immune mechanisms may have been not involved in the patients in whom GPI-deficient Grs remained miniscule. Given that mPNH-GPs were undetectable in AA patients possessing HLA(-) Grs and in many patients with long-standing AA refractory to IST, the presence of mPNH-GPs may be a marker of oligo- or poly-clonal hematopoiesis, which can be eliminated by the clonal expansion of HLA(-) HSCs or some other HSCs with intrinsic abnormalities in patients with long-standing AA, findings that are compatible with the absence of mPNH-GPs at the diagnosis in a CML patient. Lastly, the presence of mPNH-GPs without expansion in PRCA patients responding to CsA suggests that immunopathogenesis of PRCA is distinct from that of CsA-responsive AA, which turns mPNH-GPs into sPNH-GPs.
Disclosures
Takamatsu:Ono: Honoraria; Bristol-Myers Squibb: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; SRL: Consultancy. Yamazaki:Kyowa Kirin: Honoraria, Research Funding; Novartis Pharma: Honoraria. Miyamoto:Takeda: Honoraria; Otsuka: Honoraria; MSD: Honoraria; Kyowa Kirin: Honoraria; Jassen: Honoraria; Astellas: Honoraria; Abbvie: Honoraria. Nakao:Kyowa Kirin Co., Ltd.: Honoraria; SymBio Pharmaceuticals Ltd.: Consultancy; Novartis Pharma K.K.: Honoraria; Alexion Pharmaceuticals, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.